Abstract
Background: Copy number gain and/or MYC induction leads to increased expression of miRs in the miR-17~92 cluster. Development of Burkitt lymphoma (BL) is associated with translocation of MYC resulting in MYC expression and frequent recurrent copy number gains of chromosome 13q containing the miR-17~92 cluster of miRs. MiRs in the miR-17-92 cluster, including miR19 and miR17, have been associated with B-cell lymphomagenesis. Copy number gain of 13q and high expression of miR-17 have also been associated with possible inferior outcome in children with BL. MiR-17~92 miRs can decrease expression of the PI3K inhibiting protein PTEN leading to activation of the PI3K/AKT pathway, a pathway implicated in BL lymphomagenesis. These miRs also target the pro-apoptotic protein BIM, suppression of which has also been associated with chemoresistance in BL.
Objectives: Investigate the in vitro effect of altered miR-17-92 expression on chemoresponsiveness in BL cells.
Methods: MiR expression was determined by qPCR. The miR-17-92 locus was deleted in Raji cells using custom CRISPR-Cas9 lentiviral vectors. Single cell-derived clones, R5 and R24, were established and locus deletion determined by genomic DNA PCR. Protein expression and Caspase cleavage was assessed by western blotting. Apoptosis induction was determined by flow cytometry for Annexin V-propidium iodide staining. Cell viability following exposure to chemotherapy was determined by AlamarBlue assay. SCID mice were inoculated with Raji or R24 cells by tail vein injection and survival determined by Kaplan-Meier analysis. Hind limb paralysis was used as an end-point for survival. Antagomir targeting miR17 was transduced into Raji cells by electroporation.
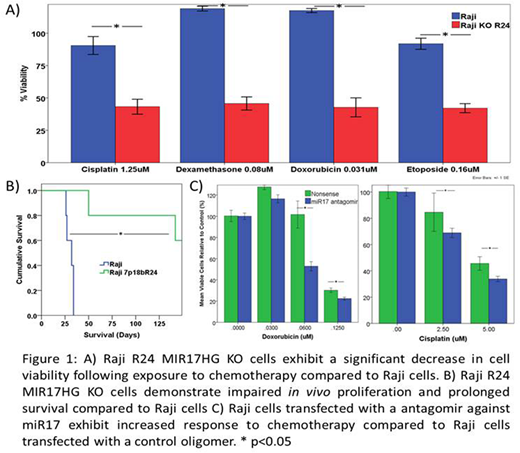
Results: Chemotherapy resistant Raji 4RH cells exhibit increased miR17 and miR19 expression and increased PI3K/AKT pathway activation compared to parental Raji. R5 and R24 Raji KO lines exhibit decreased miR17 and miR19 expression and increased expression of miR targets PTEN and BIM. When xenografted into SCID mice, R24 cells demonstrated a significant prolongation in survival compared to Raji (Raji vs R24: median 29 days vs not reached at 150 days, P<0.05) (Figure 1B). A significant decrease in [IC50] in R24 cells compared to Raji was observed following 48h exposure to doxorubicin (Raji vs. R24: 65±23nM vs 12±2nM), dexamethasone (1579±10e5nM vs 49±57nM), etoposide (571±106nM vs 106±22nM) or cisplatin (2.5±0.7uM vs 0.96±0.3uM) (Figure 1A). Raji R24 cells also demonstrate an increase in apoptosis induction following exposure to doxorubicin or etoposide. To investigate the effect of direct miR targeting, Raji cells were transfected with an antagomir targeting miR17 resulting in decreased viability following exposure to doxorubicin or cisplatin compared to Raji cells transfected with a control oligomer (Figure 1C). Antagomir transfected Raji cells express higher levels of PTEN and BIM compared to control Raji cells.
Conclusion: High expression of miR17-92 cluster miRs is associated with in vitro chemotherapy resistance. Knockout of the MIR17HG gene in BL cells results in impaired proliferation, impaired in vivo engraftement with prolonged survival in SCID mice and increased in vitro chemoresponsiveness associated with an increase in expression of PTEN and BIM. Targeting of miR17 using an antagomir approach resulted in increased PTEN and BIM and increased chemoresponsiveness. These findings highlight the role of MYC-associated miRs in in vitro chemoresistance in BL cells warranting continued investigation as a possible therapeutic target for relapsed/refractory BL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.